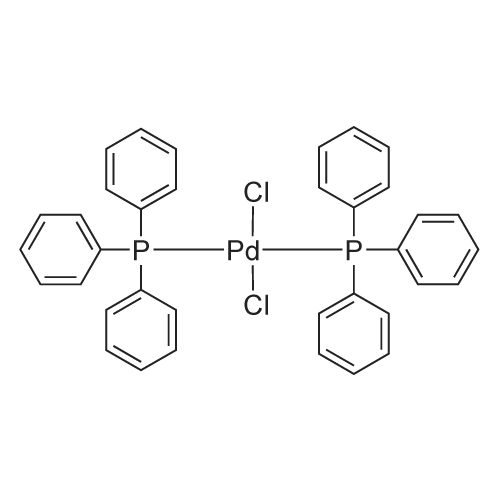
ARL892 13965-03-2
| CAS Number: | 53189-26-7 |
|---|---|
| Product Code: | ARL7736 |
| Molecular Formula/Molecular Weight: | / 673.52 |
| Purity: | 98% |
| HSN Number: | 38249090 |
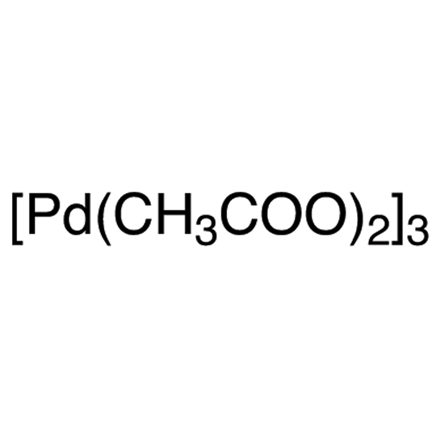
Note: For research use only, not for direct human use.*
| Pack Size | Unit Price | Stock in Mumbai WH | Stock in Hyderabad WH | Quantity |
|---|
Description : Palladium(II) acetate trimer typically appears as a reddish-brown to dark brown crystalline powder. It is odorless and has a solid, stable form under ambient conditions. The compound is moderately soluble in organic solvents such as acetic acid, chloroform, and acetone, and is commonly supplied in airtight, moisture-resistant containers to maintain its stability.
| Appearance | Brown to dark brown to amber crystalline powder |
| Purity(Chelometric Titration) | min. 98% |
| Confirms Structure | IR |
| Melting Point | 220 °C |
| Solubility | Soluble in Acetone |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | 15°C |
| Conditions to Avoid | Moisture sensitive |
| Storage Condition | Store under inert gas, in a cool, dry, and well-ventilated area, keeping the container tightly closed to prevent contamination. |
| Metal content | 48% |
| Packaging and Container | Glass Bottle |
Pictogram :

|
| Hazard Classification: Harmful if swallowed or inhaled. May cause skin and eye irritation. Handling: Use in a well-ventilated area with appropriate personal protective equipment (PPE) including gloves, safety goggles, and lab coat. |
Palladium(II) acetate trimer is a highly effective catalyst widely used in modern organic synthesis. It is especially valuable in cross-coupling reactions such as Suzuki, Heck, and Sonogashira, which are essential for the development of pharmaceuticals, agrochemicals, and advanced materials. In addition to its role in cross-coupling chemistry, this compound also finds application in the synthesis of conductive polymers, nanomaterials, and the construction of complex organic molecules. Compared to its monomeric counterpart, the trimeric form offers superior stability and reactivity, making it the preferred choice in many synthetic processes. Its enhanced catalytic performance is attributed to its unique trimeric structure, which contributes to improved efficiency and reliability across various reaction conditions.

ARL892 13965-03-2
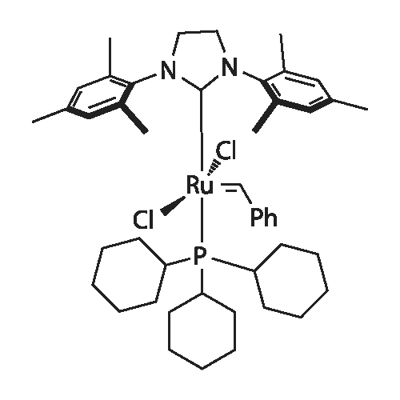
ARL6866 246047-72-3
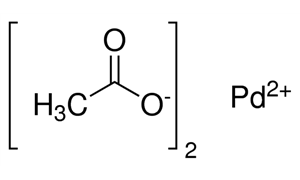
ARL8823 3375-31-3
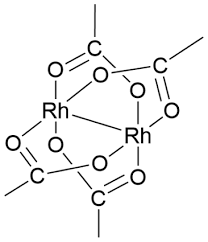
ARL6527 15956-28-2
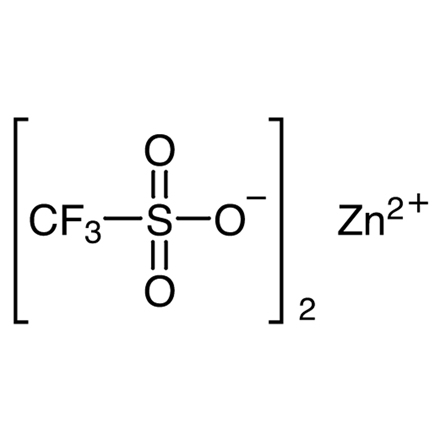
ARL8632 54010-75-2
ARL8077 14898-67-0
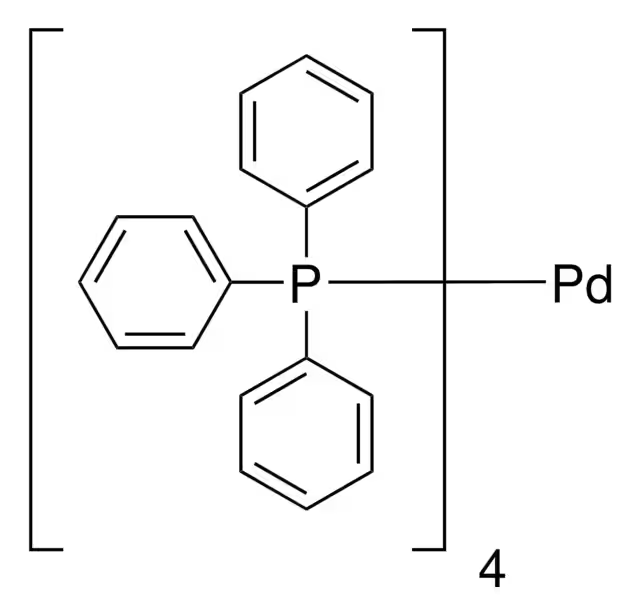
ARL7741 14221-01-3
ARL8576 51364-51-3
ARL9913 35138-22-8
ARL9914 134524-84-8
ARL9089 12354-85-7
ARL6217 12092-47-6
ARL7730 7647-10-1
ARL6527 14694-95-2

ARL7732 7440-05-3

ARL7731 7440-05-3
ARL8821 12135-22-7
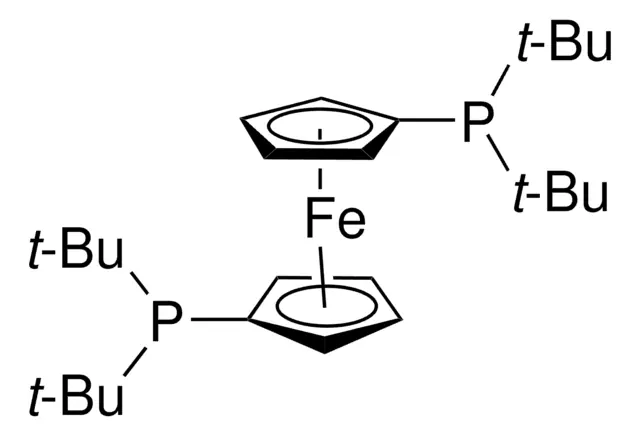
ARL4326 84680-95-5
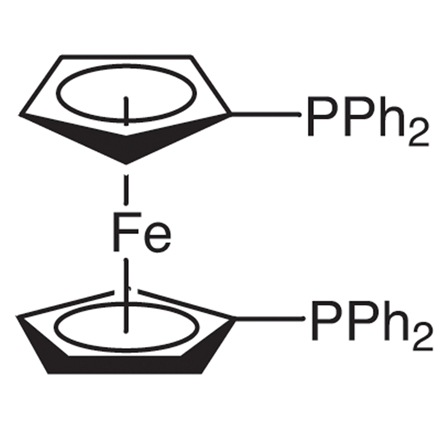
ARL4723 12150-46-8
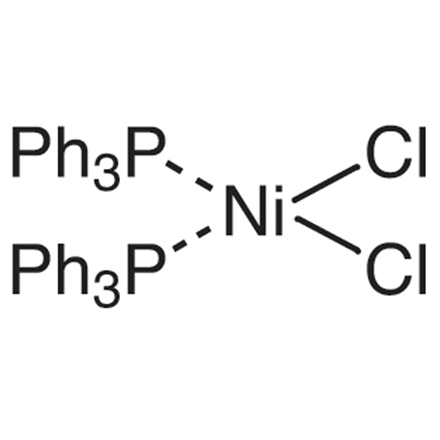
ARL1062 14264-16-5
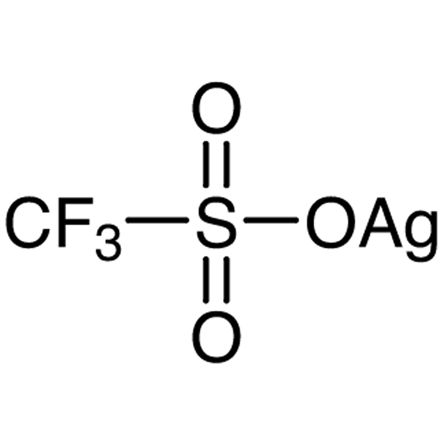
ARL8119 2923-28-6

ARL1050 14592-56-4
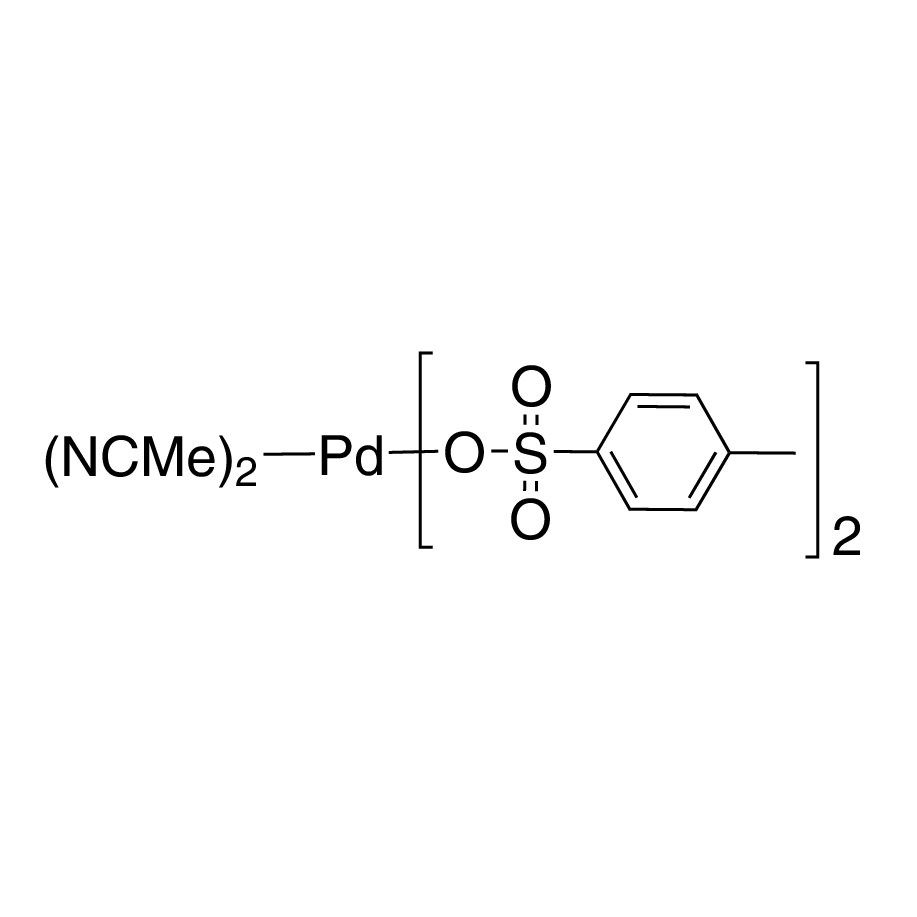
ARL9928 114757-66-3

ARL7869 1314-15-4
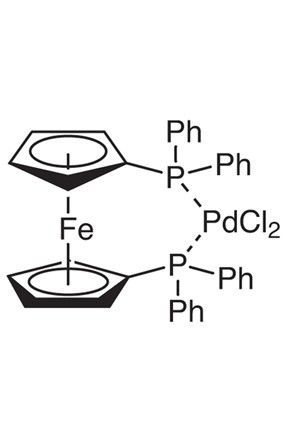
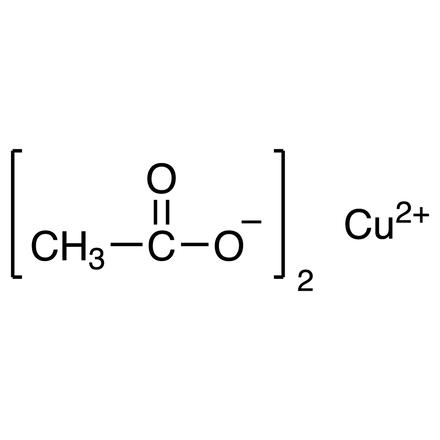
ARL6290 142-71-2
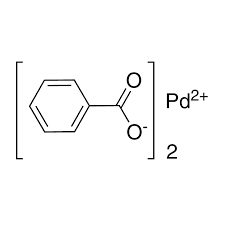
ARL10104 3375-32-4
This is a sample COA and may not represent a recently manufactured lot of the product.